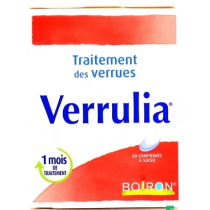
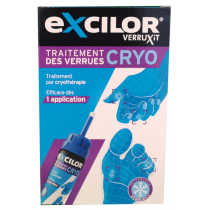
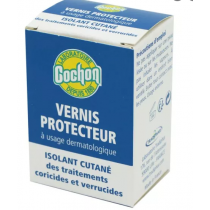
warts
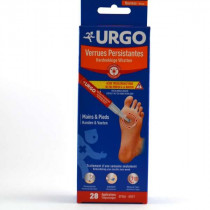
Resistant Warts - Pen - UrgoThanks to its powerful acid, the Urgo pen eliminates the wart in just one week.
Price
€15.76
There are 8 products.
Active filters